As promised on Friday, here is some initial data for this idea. I still don't know if it truly is an effect of altitude or just denature temperature, but here is what I did. I set up 8 identical reactions (10uL each) to amplify an 800bp product from pUC19 and ran them under three different cycling conditions on the same cycler on the same day (barometer = 30.43 in Hg, corrected boiling point = 93.38C). I note that there was a lot of condensation present on the upper portion of the wells following Cycle 1, but much less after Cycle 2 or Cycle 3.
Cycle 1
1) 95C 1 min
2) 95C 30s
3) 55C 30s
4) 72C 45s
5) goto 2, 29X
Cycle 2
1) 90C 1 min
2) 90C 30s
3) 55C 30s
4) 72C 45s
5) goto 2, 29X
Cycle 3
1) 90C 1 min
2) 80C 30s
3) 90C 30s
4) 55C 30s
5) 72C 45s
6) goto 2, 29X
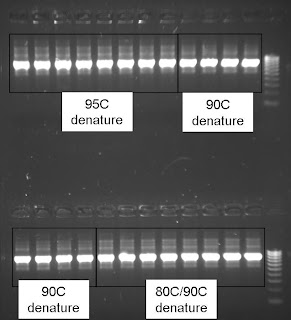
I ran 2uL each product on a gel:
Everything looks about the same, but you might notice there are more large (greater than 800bp) non-specific products in the 95C denature and the 80C/90C denature programs than the 90C denature alone. I think this is partially because 30 cycles may have been too many for this amplicon. I hope to rerun this analysis with fewer cycles another day.
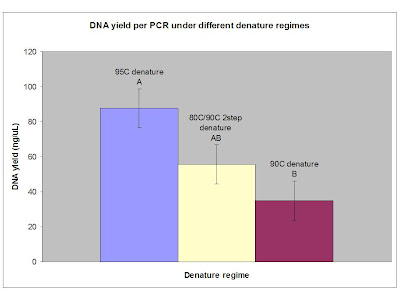
That said, significance was achieved (ANOVA p = 0.0104)! Again, I think this is largely due to the added non-specific products. Here is a nice bar graph showing the results with Tukey's HSD separations denoted. The concentrations were obtained using OD260 method following ethanol precipitation of all products. Each sample was resuspended in 50uL 1X TE.
What can I take away right now? 90C is adequate for denature of a simple bit of DNA such as pUC19 and I know it avoids boiling my sample at this altitude. Less condensation appears on the upper portion of the tube walls under a 90C denature, presumably preventing concentration of reagents which can lead to production of non-specific bands. The effect, regardless of its cause, results in a significant difference in total PCR yield, but presumably more of the product in the 90C denature is the product I want while the other cycles produce unwanted by-products.
Where to go next? Repeat with fewer cycles, possibly use a qPCR to guide the appropriate number of cycles, measure with a pipet the volume of reaction that remains at the bottom of the well following each cycle next time. For now, reduce denature step to 90C. When this particular reaction works a bit better, test it again at different altitudes. Also maybe test a more complex target.
At the very top of Snowbowl (~11,500ft, if they will loan me an outlet for the day), the boiling point would be 89.2C on the same day, thus boiling all of my reactions. In the Verde valley (~3,500ft), the boiling point would be 96.9C thus eliminating all boiling for all reactions.
A colleague told me this weekend his new motto is "free Tibet (to do PCR)!"
Monday, January 30, 2012
Friday, January 27, 2012
High altitude PCR
It occurred to me the other day that some unreliability among PCR samples that we experience here in Flagstaff may be attributable to the altitude here (7000ft). I did some googling on high altitude PCR but came up with pretty much nothing. Our lab has 4 Biorad iCyclers, 1 Biorad iCycler for qPCR, 1 BioRad Tetrad (MJ style with 4 blocks), 1 MJ Research PTC-200, and 2 MJ Research PTC-100s.
I first dug into the manuals to try to find anything about altitude correction factors. Nothing. The closest I came was finding that they could be "safely used" at altitudes up to 2000m (~6500ft). That gets almost all the way up to Flagstaff, but says nothing about whether there are any altitude-related issues I should be thinking about.
I then called BioRad tech support and chatted with a rep for a bit on this issue. She was a little amused and quickly regurgitated the ideal gas law (PV=nRT) which would have taken me a little longer to come up with. She pointed out that there are temperature sensors in all of the blocks of the machines we use, so the temperature reported by the cycler is correct as long as you accurately enter the volume of your reaction so it can correctly calculate the sample temperature from the block temperature probes.
I then pointed out that water boils at just under 93C here in Flagstaff (assuming barometric pressure of 23 in. Hg). If we are experiencing high atmospheric pressure (most of the time), the boiling point is slightly higher (closer to 95C) or if we have a storm (low atmospheric pressure) it could go as low as 91C. What this means is that every person here at NAU who follows the prescribed protocol for their PCR is boiling their reactions at every denature step. Since the denature temperature for most programs is still 94-95C, this is just the cusp of the boiling point on most days (it is usually sunny here). It also is not above the magic point at which you will significantly degrade the half-life of your enzyme with each step (about 94-95C). The boiling can still have a significant effect on the reaction by changing the concentration of your buffer (increased concentration of all reagents). Sometimes the moisture is retained due to the heated lid preventing condensation on the upper part of the tube and the lid compression preventing any gaseous escape, but more often than not, your reaction will contain less fluid at the end of the cycle than you began with.
Example: If you perform 10uL reactions and after 35 cycles you have 8uL, your MgCl2 concentration (to pick a reagent) will increase from 2mM to 2.5mM over the course of your cycle. Excess of MgCl2 can contribute to mis-priming and production of non-specific products. Excess KCl can produce unwanted short non-specific products. Excess polymerase can result in all kinds of background (a smeared appearance). Some environmental DNA samples such as are often processed in our lab also contain a lot of PCR inhibitors (e.g. polyphenols). The evaporation can therefore increase the endogenous inhibitor concentration to a point that effectively stops the reaction somewhere along the cycle.
You get the idea. Evaporation BAD!!!(say it like Dana Carvey playing George HW Bush) Though she couldn't provide me a concrete solution to this concern short of an artificially pressurized laboratory, she offered two suggestions that sounded very good to me:
1) Persons performing PCR at high altitude should reduce their temperature of denaturation according to the local atmospheric pressure. For our altitude, she suggested 90C. I asked if this time should be extended slightly to account for the lower temperature and she said she did not know, but it probably wouldn't hurt...which takes us to her second suggestion.
2) PCR (generally speaking at all altitudes) of GC-rich regions should include a "pre-denature" step of 80C for 1 min to "slow" the denature step to allow for the disentanglement of complex secondary structures and eventual denaturation of the GC-rich region. I should point out that she offered this as a complete alternative to ever adding DMSO to your reaction which, as you may know destabilizes hydrogen bonding, thus allowing efficient denaturation of GC-rich regions, but then also complicates your annealing step. She claimed the 80C for 1 min will solve this problem without messing with your annealing and thus may also be useful for high-altitude PCR.
What I learned: At 7000ft, reduce the annealing temperature to 90C. I can probably keep my denature time at 20-30 sec, but a pre-denature step of 15-30 sec at 80C may improve overall reaction efficiency with no further changes (longer if PCR target region is GC-rich).
Happy high altitude PCRing!!
Friday, January 13, 2012
Reducing laboratory costs
I was chatting with a colleague this morning about cutting lab costs. Rather than go into a lengthy discussion, I thought I would post a quick list of some things I do that bake a big difference in my lab budget.
- Use chemical methods when possible for DNA extractions rather than kits. Kits often cost $2-4 per sample. By chemical method in 2mL tubes, the cost immediately plummets. If you scale down your extraction into 96-well PCR plates, your reagent needs as well as your labor get minimized. Some of us just need a few PCR reactions. Depending on your organism, you may be able to do a rapid alkaline lysis method, further reducing your costs and material demands (e.g. http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CFsQFjAB&url=http%3A%2F%2Fwww.biotechniques.com%2Fmultimedia%2Farchive%2F00010%2F03344rr04_10933a.pdf&ei=98wQT7uZMam02gXiyIGECg&usg=AFQjCNF1HORNnYJQdbCmzbNYj-QGG0oJTw&sig2=lw4xwfnlpUgXNKVE3PPVrw).
- Reduce your PCR reaction volume. Most PCR kits suggest a reaction volume of at least 25uL, some as large as 100uL. I almost never do more than 10uL. If using a thermal cycler with adequate lid pressure, 6uL is possible in a 96 well plate. I now use 384-well plates and with the smaller wells (less headspace), I get good reactions of 4uL. That leaves me 2uL to run on a gel and another 2uL for capillary analysis. No waste!!
- Use reusable silicone PCR mats (e.g. http://www.phenixresearch.com/products/mpcs-3510-sealing-mat-pressure-fit-lid.asp). These things are great and I have always enjoyed less evaporation than with for instance, microseal B films. You can bleach or autoclave them if you like, but that will reduce their life. I simply throw used mats into a beaker with hot tap water for 10 minutes, rinse with RO water, and dry on a paper towel. I've never had a problem with my NTC reactions. For 384-well plates, try these: http://www.phenixresearch.com/products/smx-pcr384-sealing-mat.asp
- Use PCR plates (96 or 384) rather than strip tubes. Each plate costs about as much as 4 strip tubes last I checked. You don't have to throw out your PCR plate after running just a few reactions either. I keep partially used ones on my bench and just mark off the already used wells with a marker. When I need new wells, I peel back my silicone mat and use the unused wells, and don't bother to clean the mat until every well has been used. Still no problems with my NTCs.
- Agarose gels are reusable. Once or twice, but it can get pretty ugly. Make sure to keep spent gels intact in a sealed container (can hold numerous gels at once). Break the desired size of a gel into a flask for remelting and bring the mass back up where it should have been with RO water (if you had a 80mL gel, it should weigh ~80g). Add some new dye (e.g. ethidium or SYBR), pour, and run as always. You will notice some increased background, but for a quick gel to check some unimportant results, this works fine.
- SYBR safe can be used at 25,000X rather than 10,000X with no real loss of sensitivity.
- BigDye reactions (10uL) work fine with just 0.5uL BigDye. Add 2uL sequencing buffer, and sequence as before, or with this modified protocol I like: http://www.biotechniques.com/multimedia/archive/00003/BTN_A_000112499_O_3096a.pdf
Of course there are other things you can do, but this is all I have time for today...
Tuesday, January 10, 2012
Bad water!!
For my PhD project I have developed a set of microsatellites for pinyon pines, but have been unable to publish due to lack of consistent chemsitry. For a while I thought I was being sloppy, but after washing my pipets and the lab bench and redoing a set of reactions, my results kept getting worse. Eventually, I traced my problem to the flask of water that I use when making reagents and PCR reactions. I took the appropriate measures
of getting a new flask, autoclaving fresh Nanopure water and starting fresh. Everything worked beautifully! Then two weeks went by and I was back to the same miserable non-results. Failed reaction after failed reaction, I kept repeating my work since every 10th reaction or so would still function.
Eventually, I did some field work and collected fresh tissue and extracted the DNA from this tissue. Spectrophotometer readings on each sample are mostly spectacular (DNA concentrations of ~150-250ng/ul, 260/280 of ~1.95, 260/230 of ~1.95). I
tried PCR amplification from this new tissue (full strength and 1/10x dilution) and got the same lame result (no desired products, a few random PCR artifacts). Just to be safe, I repeated the PCR but this time using 1/10x DNA diluted in Tris and a "lazy-mans dilution" to 1/50x. Basically, you set up 10uL reactions, aliquot 9uL master mix to each well, add 1uL template DNA to the first set of reactions, mix and spin down, and add 2uL the
first set of reactions to the second set of reactions - voila, 1/50x dilutions with no wasted DNA sample!! I had a few successes, but it no patterns emerged that could help me to consistent success (Gel 1, below)
So I figure I have primer degradation after just 3 weeks. Since I work with pines, notorious for their endogenous suite of PCR-inhibiting compounds, I wondered the other night while falling asleep if I could simply include polyvinylpyrrolidone (PVP) in my PCR reactions to sequester polyphenols that might be contributing to reaction failure. When I got to lab the next morning, I googled the concept and found a paper on just such a thing (http://nar.oxfordjournals.org/content/27/3/915.abstract). So
I prepared a set of of new 1/10x DNA dilutions, this time using my handy stock of PVP-40 rather than Tris to make the dilution. I repeated the 1/10x and 1/50x lazy mans dilution from Gel 1 and things suddenly worked and made sense (Gel 2). As I have long known and practiced, diluting your DNA can reduce the effects of PCR inhibitors in your sample, but the inclusion of PVP can enhance your reaction even more. In gel 2, the first locus (top comb) is more difficult to amplify, (1/10x DNA, no PVP = no PCR product), but at the highest dilution factor and with the inclusion of PVP I get a success of 3 out of 4 reactions. The lower comb, and the easier to amplify locus also gives increasing success in the same way.

So what did I learn? PCR amplification from pines can be enhanced by the inclusion of about 1% PVP-40 in your PCR reaction. You can simply dilute your DNA in PVP-40 rather than adding one more thing to your PCR reaction mix. Initially my water had gone bad, resulting in degradation of PCR primers in a relatively short amount of time. I immediately checked on our Nanopure filtration system and found evidence of bacterial growth in the filters. Probably we should service our filtration device and in the meantime purchase "molecular grade" water from a reputable vendor. While I absolutely hate purchasing water, I hope that this can serve as a lesson to more than just myself.
Happy PCRing!!
Tuesday, January 3, 2012
Hello!
My name is Andrew Krohn and this is my first day as the Research Technician for Northern Arizona University (NAU) Environmental Genetics and Genomics Laboratory (EnGGen). I am taking over this position from the tireless Tamara Max who has moved on to an enviable position with Paul Hohenlohe ( http://webpages.uidaho.edu/hohenlohe/index.html). I am initiating this blog so that I can quickly post about topics of interest to the research community at NAU and elsewhere. I hope to include experiences, molecular biology tips and tricks, cost-saving measures, and other lab-related information.
I am in the 3rd year of my PhD here at NAU. My research focuses on pinyon pines and associated soil microbial communities. Recently I have developed a set of microsatellite markers for use in pinyon pines (publication pending soon). For soil microbial analysis I use tRFLPs to screen samples in volume and next-generation sequencing for more thorough analyses. I work primarily with DNA and perform a lot of PCR.
As a first step, I have updated the contact info at the EnGGen website and corrected the link to the EnGGen webcalendar wherever I found it broken.
EnGGen website: http://www.enggen.nau.edu/index.html
EnGGen contact info: http://www.enggen.nau.edu/staff.html
Additionally, I try to maintain a professional website for myself here: http://dana.ucc.nau.edu/~alk224/AKrohn/index.htm
Subscribe to:
Posts (Atom)